For systemically identifying the potential key regulators controlling the fruit-ripening process, we firstly reported the method on how to use the frontier mAbArray chip technology which was originally applied in human medicine, as an effective tool to identify the key regulatory proteins.
Strawberry fruit ripening involves a series of physiological and biochemical changes, including the enlargement and softening of fruit, the increase in sugar, and changes in texture, color, flavor, and aroma. However, an important aspect of fruit ripening is the accumulation of a diverse array of secondary metabolites. The precursors for their metabolism would be produced early in strawberry fruit development and later in the ripe stage these compounds would provide the consist for the fruit to become and remain attractive. Most consumer from Asia would like to eat sweetness and strong flavor. Therefore, high-content of sweetness is an important target for strawberry breeding in China. Sucrose is a key signal of strawberry ripening and plays an important role in its regulation. Thus, it is of great significance to study the key regulatory proteins of sugar accumulation during the strawberry ripening process to promote high-sugar strawberry breeding.
Based on the morphological changes in developing strawberry fruits, six stages of fruit development (Figure 1 receptacle, small green fruit stage, big green fruit stage, white fruit stage, half red fruit, and fully red fruit) were sampled for crude protein extraction. Proteins were extracted from fruit using the tricarboxylic acid (TCA)/acetone precipitation and SDT cracking method according to a published protocol (Figure 2).
As we all know, antibodies are essential for elucidating the function of genes decoded by genome sequencing. However, an effectively technology for proteome-scale antibody generation in strawberry has been developed not too much. However, the ripening process in the non-climacteric model fruit strawberry is mainly regulated by the phytohormone ABA instead of ethylene, and an affordable technology for proteome-scale antibody generation has not yet been developed.
In our study, the Proteome Epitope Tag Antibody Library (PETAL) and its array were developed to identify the key proteins during strawberry fruit ripening. Analysis of the entire proteome from strawberry fruits of the cultivars ‘Zijin Jiuhong’ and ‘Tokun’ at six key development stages (from receptacle to fully red fruit) enabled construction of a PETAL consisting of 7,000 monoclonal antibodies (mAbs) against 8,954 proteins. Screening in these mAbs using an array chip, 846 differential antibodies were chosen for western blot (WB) identification, and 123 qualified antibodies were selected. Gas Chromatograph-Mass spectrometry (GC-MS) analysis of different stages of strawberry fruit development showed that 18 proteins were identified and most proteins were major members in sugar or acid metabolism, plus other primary energetic metabolism (Figure 3). Crucially, our microarray assays indicated that some pivotal proteins including alcohol dehydrogenase class-3, fructose-bisphosphate aldolase, phosphoglucomutase, and their target proteins were related the regulation of fruit ripening.
By further digging the key proteins in modulating the strawberry sugar accumulation. DNA methylation is jump out by comprehensive analysis. According to the former study reported that DNA methylation is involved in many developmental processes in plants. RNA-directed DNA methylation (RdDM) was significantly downregulated during strawberry fruit ripening, resulting in demethylation in the strawberry genome. In our study, the mAbArray chip analysis together with the subsequent large sample WB validation indicated that the key proteins involved in DNA methylation, such as MET-hm and SAM 2, which play their corresponding important roles in the process of DNA methylation, may also play important roles in the regulation of sugar accumulation, especially during the late stages of strawberry fruit development (Figure 4). In particular, protein expression, which is closely related to methylation, showed a negative correlation with sugar accumulation in the late stage of strawberry ripening.
In conclusion, we suggested that DNA methylation may be the key factor in regulating sugar accumulation and fruit ripening in strawberry. Future combined analyses including other omics, such as metabolomics or proteomics, could provide a more systematic and accurate process to achieve experimental results. This work was directly supported by the National Key Research and Development Program of China (2019YFD1000800).
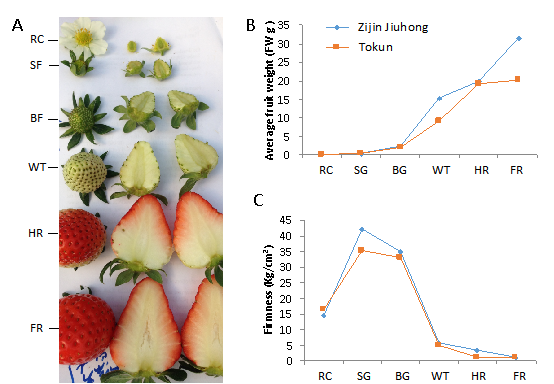
Fig. 1 The changes in phenotype, average fruit weight, and firmness during strawberry (Fragaria × ananassa ‘Zijin Jiuhong’ and ‘Tokun’) fruit development. (A) Different developmental stages. The development process can typically be divided into six stages (receptacle: RC, small green fruit: SG, big green fruit: BG, white fruit: WT, half red fruit: HR, and fully red fruit: FR). (B–C) Changes in average fruit weight and firmness throughout fruit development in Zijin Jiuhong and Tokun cultivars. The stages labeled on the X-axis correspond to those presented in (A).
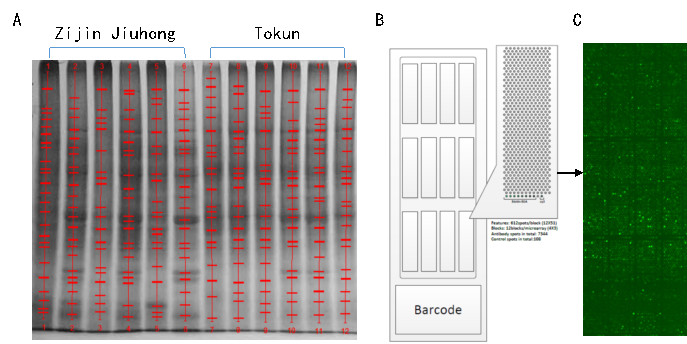
Fig. 2 Construction of the strawberry mAb library and screening of differentially expressed proteins in ripening fruit with a mAbArray chip. (A) Strawberry protein antigens used to generate the mAb library. SDS-PAGE analysis of proteins extracted from strawberry fruit that were used as antigens for antibody library construction. Proteins were extracted from the fruit of the Zijin Jiuhong (lane 1–6) and Tokun (lane 7–12) cultivars at the ripening stages of receptacle, small green fruit, big green fruit, white fruit, half red fruit, and fully red fruit. The proteins in 20 µg of strawberry were separated by SDS-PAGE and stained with Coomassie blue. (B) A model of the antibody chip analyzed by hybridization. Antibody chip after hybridization with Cy5-labeled goat anti-mouse IgG, and scanning for the Cy5 signal. (C) Magnified image of one block. The light spots indicate the positive controls (biotin-labeled BSA), and the dark spots indicate the negative controls with no significant signals.
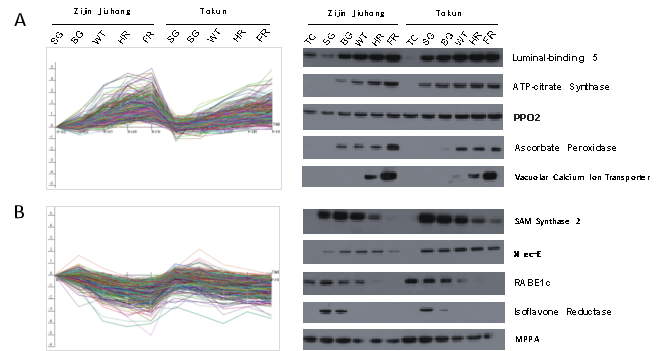
Fig. 3 The typical proteins with positive (A) and negative (B) expression modes, and their corresponding WB identification results (right) during the strawberry fruit ripening process.
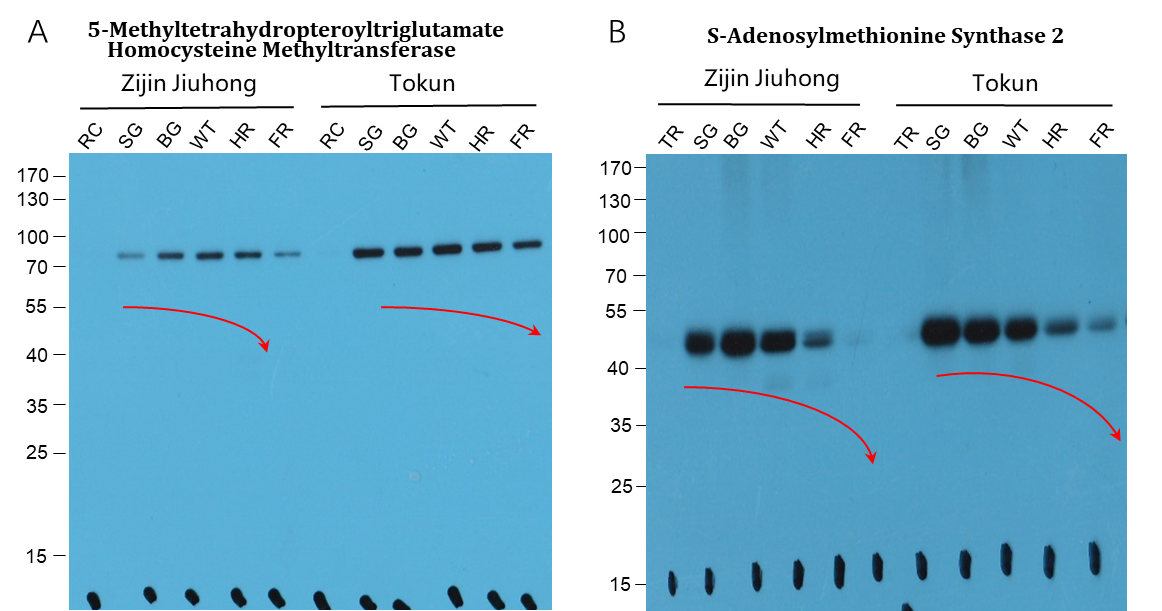
Fig. 4 WB identification of typical differential expression proteins in Zijin Jiuhong and Tokun large sample validation.


